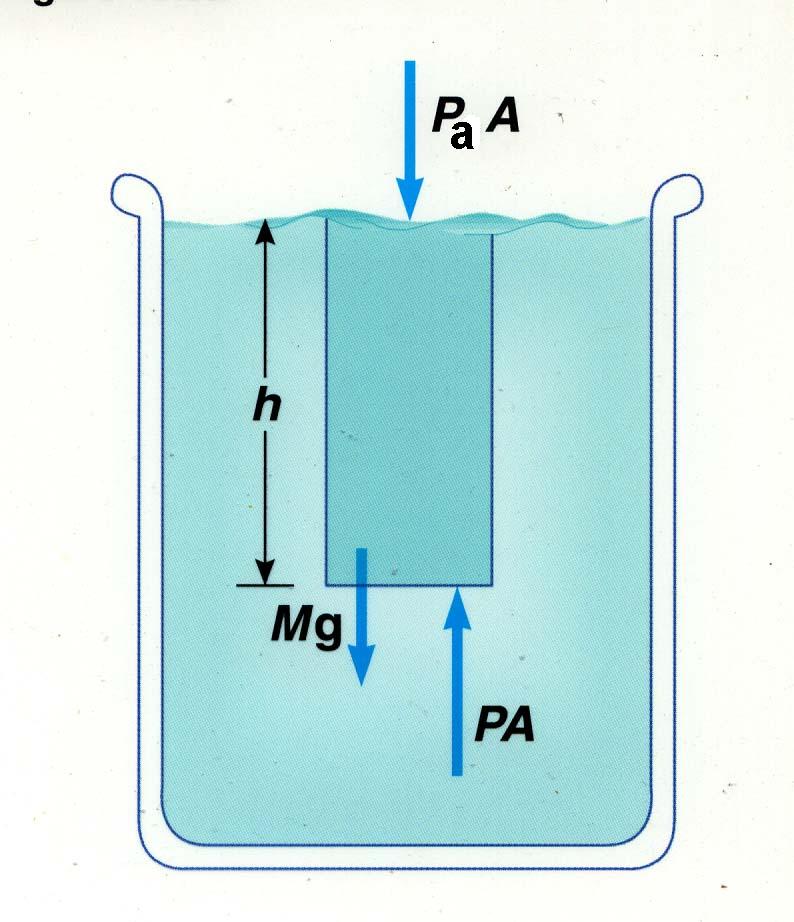
 |


Notice that ice has a lower density than either water (freshwater) or seawater (saltwater), so it will float in them. Seawater, however, has a higher density than freshwater, which means that the seawater will sink when it comes in contact with freshwater. This behavior causes many significant ocean currents and a concern of glacier melting is that it will alter the flow of seawater, not just add more water to the ocean -- all from the basic functioning of density.
To convert the density to grams per cubic centimeter, merely divide the values in the table by 1,000.
| Material | Density (kg/m3) |
| Air (1 atm, 20 degrees C | 1.20 |
| Aluminum | 2,700 |
| Benzene | 900 |
| Blood | 1,600 |
| Brass | 8,600 |
| Concrete | 2,000 |
| Copper | 8,900 |
| Ethanol | 810 |
| Glycerin | 1,260 |
| Gold | 19,300 |
| Ice | 920 |
| Iron | 7,800 |
| Lead | 11,300 |
| Mercury | 13,600 |
| Neutron star | 1018 |
| Platinum | 21,400 |
| Seawater (Saltwater) | 1,030 |
| Silver | 10,500 |
| Steel | 7,800 |
| Water (Freshwater) | 1,000 |
| White dwarf star | 1010 |